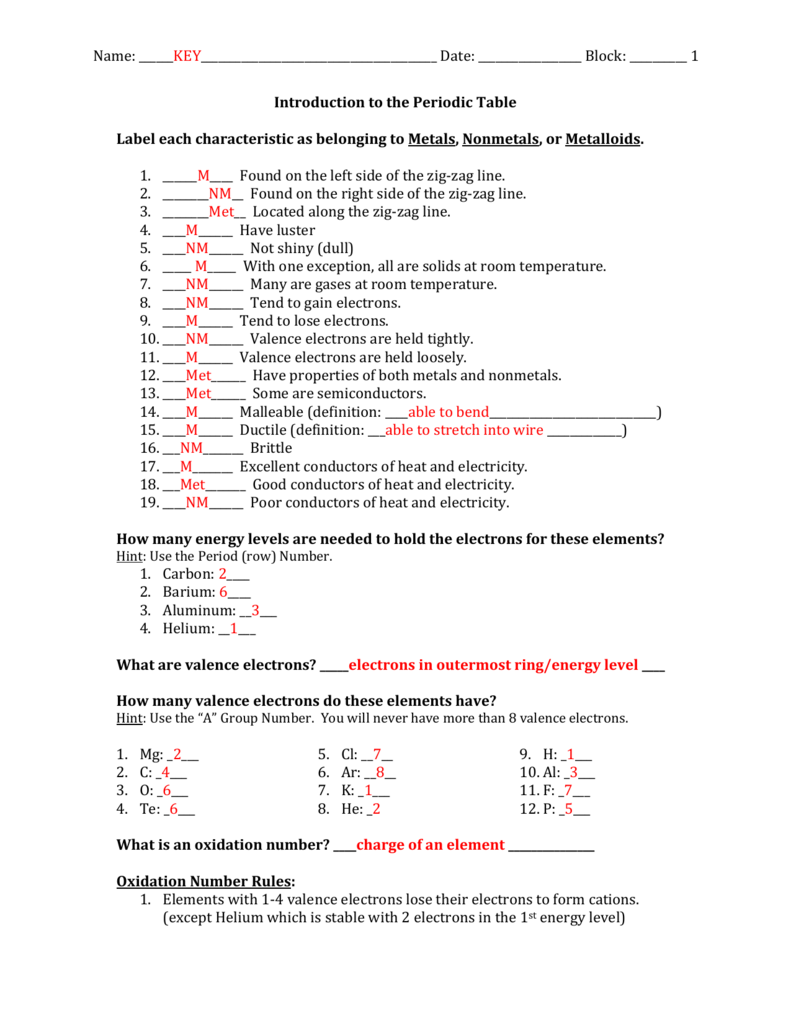
Is More Than 4 Valence Electrons a Metal or Nonmetal
They are all metals. Conducts electricity in solid state.
Is It True To Say That Metals Have 1 3 Valence Electrons And Nonmetals Have 4 8 Quora
Metals have lower electronegativities than nonmetals and they tend to have fewer than four valence electrons so they are more likely to lose electrons in order to achieve a.

. Share on Whatsapp Indias 1 Learning Platform Start. Further explanation Valence electrons are electrons used in forming bonds and are in the outer shell of an element. A case in point lead has 4 valence electrons as you could see in lead dioxide.
The non-metal is a bad conductor of electricity. Nonmetal elements have more than 4 valence electrons. Start studying metals and nonmetals.
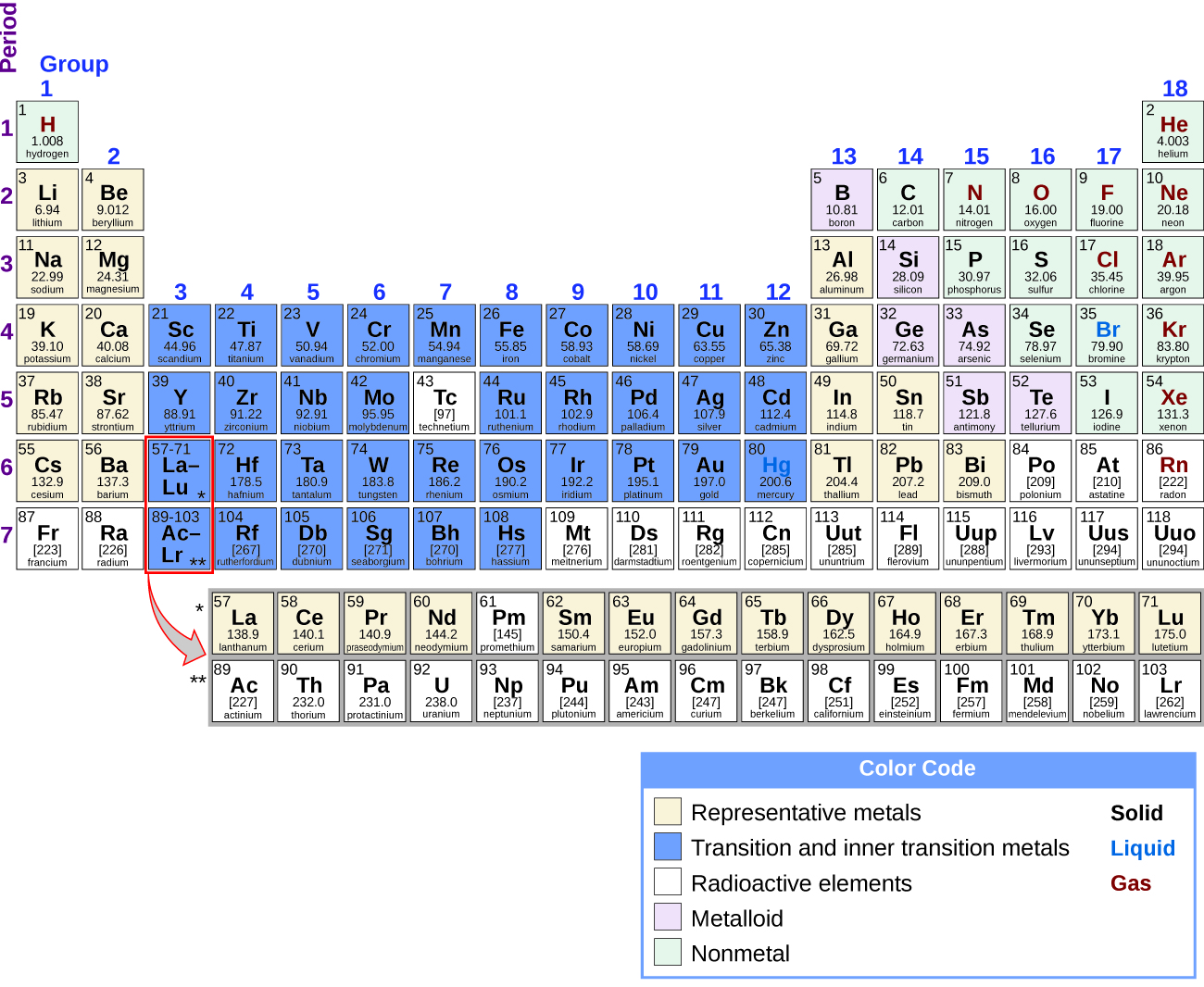
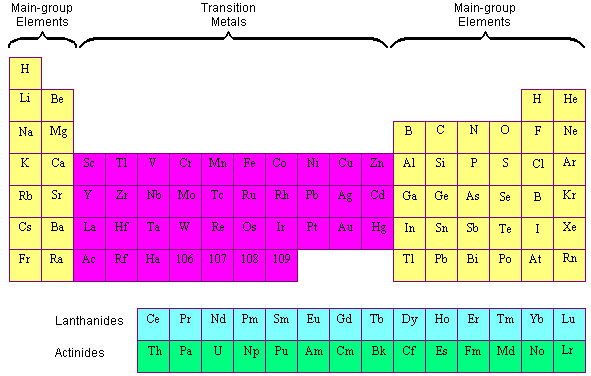
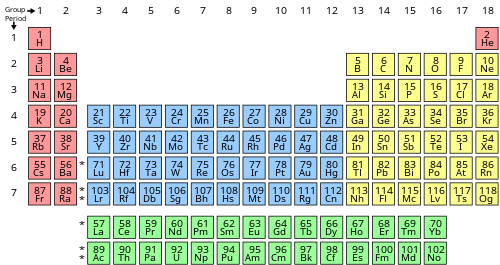
Part of the transition metal Lanthanides and actinides. Metal elements have less than 4 valence electrons. When the number of valence electrons in the atom is more than 4 the element behaves as non-metal.
There is a correlation between the number of valence electrons and the ability of a solid element to conduct electricity. The element that has a less than 4 valence electrons is a metals. The non-metals are a bad conductor of electricity.
The element that has a less than 4 valence electrons is a metals. Form oxides that are basic. Usually have 4-8 electrons in their outer shell.
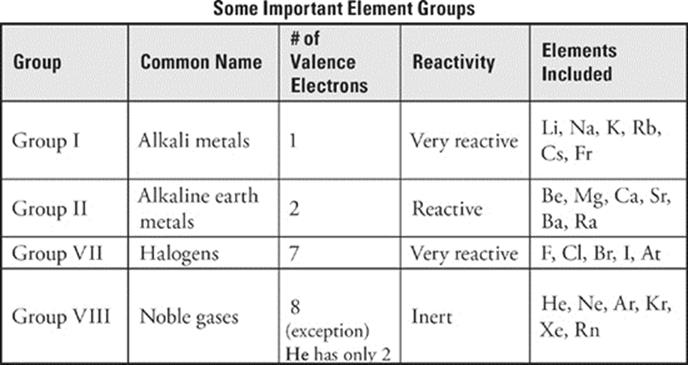
Are good reducing agents. A binary ___ compound is a compound in which one element present is a metal and the other element present is a non-metal. All of the halogens.
Metals could have 4 or more valence electrons. More than 4 valence electrons. But the reason a material conducts or.
An element behaves as a non-metal when the number of valence electrons in its atom is more than four. Elements in the middle of the. To answer the question based on elements chemical properties.
The non-metal is a bad conductor of electricity. Part of the transition metal Lanthanides and actinides. Nitrogen has 5 valence.
The three well-known examples of the insulator are nitrogen sulphur and neon. When the number of valence electrons in the atom is more than 4 the element behaves as non-metal. Lose their valence electrons easily.
- between a metal atom and a non metal atom - when a metal interacts with a nonmetal it can transfer one or more of its electrons to the nonmetal - the metal atom then becomes a cation - the nonmetal atom becomes an anion - isnt really a bond more of an electrostatic attraction. Such elements or materials are called as the semiconductor. What kind of elements has more than 4 valence electrons 1 See answer Advertisement Advertisement.
The non-metal is a bad conductor of electricity. More cases are tin 4 antimony 5 polonium 6 and so on. Carbon silicon and germanium are semiconductor elements and these have precisely four valence electrons in their atoms.
Metal Elements have low. Identify the nonmetal in the second period that is more reactive than Nitrogen and less reactive than Fluorine. Prefers to receive electrons on chemical reactions.
Part of the transition metal Lanthanides and actinides. Generally speaking conductors have fewer valence electrons while insulators have more. The metalloids with less than 4 electrons in the valence shell tends to act like metals while metalloids with more than 4 valence electrons tends to act more like.
Identify the noble gas with two valence electrons. Identify the metal in period 4 that is more reactive than Calcium. While a binary ___ compound is a compound in which only two nonmetallic elements are present.
Usually have 1-3 electrons in their outer shell. Non-metals have high ionization energy. Element has more than 4 valence electrons.
Learn vocabulary terms and more with flashcards games and other study tools. The metalloids are the elements with the ability to act as both metals and nonmetal. Lead was the first metal to be ever smelted by human beings.
Identify the element that has five valence electrons and two energy levels. I hope this can help. The main group elements on the right side of the periodic table from Group 15-18 have more than four valence electrons and gain enough electrons to.
Low electronegativity indicates low ability to attract electrons. When the number of valence electrons in the atom is more than four the element behaves as non-metal. These elements and the materials made of those elements are called insulator or insulation materials.
The metalloids have wide application with its characteristic in between metals and nonmetals.

Valence Electrons Ck 12 Foundation
The Periodic Table And Bonding Subject Review Cracking The Sat Chemistry Subject Test

Ionic Bonding Metal Nonmetal Valence Electrons Vs Charge Valence Electrons Outer Shell Electrons Valence Electrons Group A Charge When An Ppt Download

How To Identify Metals Nonmetals And Metalloids On The Periodic Table Youtube

Properties Of Nonmetals And Metalloids By Group Wikipedia

Solved When Number Of Valence Electron Is More Than To 4 It Chegg Com

Predicting Bond Type Metals Vs Nonmetals Video Khan Academy

Valence Electron And Electric Conductivity Electrical4u

Valence Electrons Definition Chart Configuration Examples

Ionic Bonding Metal Nonmetal Valence Electrons Vs Charge Valence Electrons Outer Shell Electrons Valence Electrons Group A Charge When An Ppt Download

Valence Electrons Characteristics And Determination Of Valence Electrons

Comments
Post a Comment